Senescence and Mitochondria
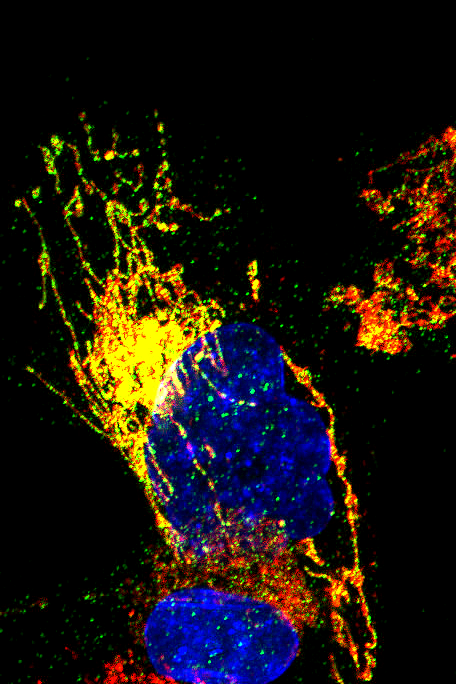
Mitochondria, the powerhouse of the cell, play a crucial role in cellular energy production and metabolism. As cells age or experience stress, mitochondrial function can decline, leading to the excessive production of reactive oxygen species (ROS). This mitochondrial dysfunction is closely linked to cellular senescence, a state in which cells permanently stop dividing and secrete inflammatory factors that can contribute to tissue aging and age-related diseases. The interplay between mitochondrial health and senescence is a key area of our research, as targeting mitochondria may offer new strategies to delay aging and improve tissue function.
Mitochondria have their own life cycle, involving biogenesis (the creation of new mitochondria), fusion and fission (processes that balance mitochondrial shape and function), and mitophagy (the selective degradation of damaged mitochondria). These events are crucial for maintaining mitochondrial health and function. However, during cellular senescence, the regulation of these processes can be disrupted, leading to an accumulation of dysfunctional mitochondria. This contributes to the enhanced ROS production and the senescence-associated secretory phenotype (SASP), which drives inflammation and further promotes aging.

The Role of GDF15 in Mitochondrial Homeostasis and Cellular Senescence
Our research has uncovered a previously unknown role for Growth Differentiation Factor 15 (GDF15), a stress-responsive cytokine also known as a mitokine, in maintaining mitochondrial health and delaying cellular senescence. By knocking down GDF15 expression in human dermal fibroblasts, we observed significant mitochondrial dysfunction and the onset of premature senescence. This was accompanied by a distinct senescence-associated secretory phenotype (SASP), which contributes to tissue aging. Furthermore, fibroblast-specific loss of GDF15 expression in a 3D reconstructed human skin model led to epidermal thinning, a hallmark of skin aging. These findings suggest that GDF15 is crucial for mitochondrial homeostasis, playing a key role in delaying cellular senescence and mitigating age-related changes in the skin.

The Role of Mitochondria in UVB-Induced Skin Aging
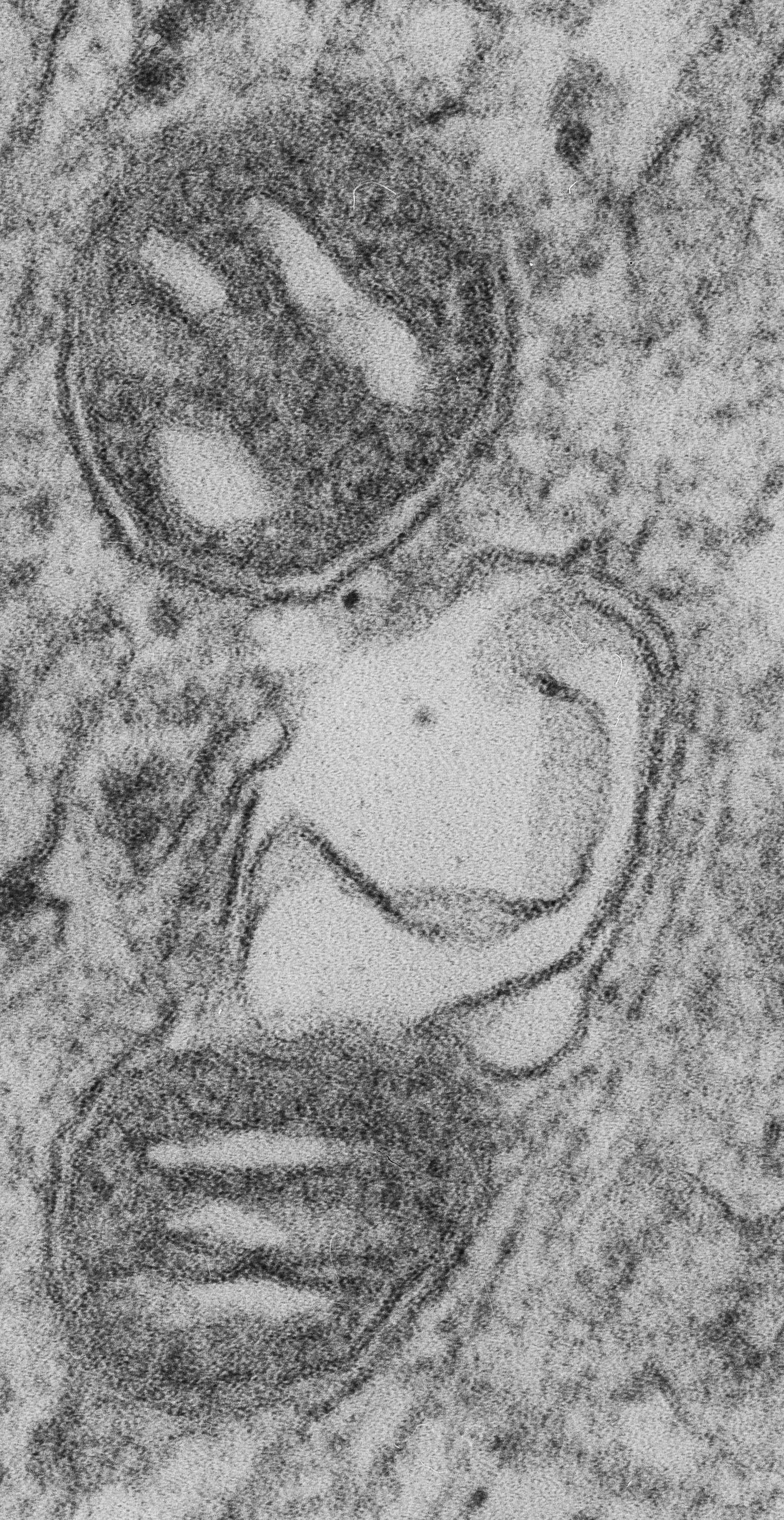

Skin aging is influenced by both intrinsic factors, such as physiological and genetically determined changes, and extrinsic factors, including exposure to sunlight, smoking, and diet. One of the primary external contributors to skin aging is UVB radiation, which causes skin injury through the generation of free radicals, oxidative byproducts, and DNA damage. The accumulation of senescent cells in the skin is a hallmark of aging, and mitochondria play a crucial role in the development of stress-induced cellular senescence. However, the specific aspects of mitochondrial function related to cellular senescence and extrinsic skin aging are not yet fully understood. Our recent research demonstrates that UVB irradiation of human dermal fibroblasts (HDF) leads to mitochondrial damage, which is counteracted by NIX-dependent mitophagy, a process essential for cell survival under these stress conditions. Additionally, we found that UVB irradiation induces the shedding of extracellular vesicles (EVs), a process that is significantly enhanced in NIX-depleted cells. These findings establish NIX as the primary mitophagy receptor in UVB-induced senescence and suggest that EV release may serve as an alternative mechanism for mitochondrial quality control in HDFs.
